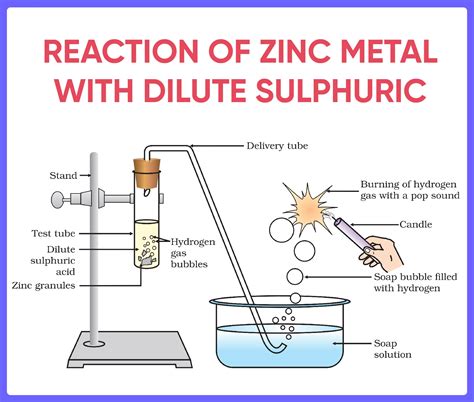
dropping zinc pallets into a test tube containing dilute h2so4|zinc and sulfuric acid experiment : bulk Zinc reacts with dilute sulphuric acid to produce hydrogen gas (H 2) and zinc sulphate. Zn(s) + H 2 SO 4 (g) → ZnSO 4 (s) + H 2 (Zinc) (dil. sulphuric acid) (Zinc Sulphate) (Hydrogen gas) A prece serve para que o amado tenha mais desejo por você. Logo, te ajuda a colocar a pessoa que ama em uma posição de abertura em relação a você. Trata-se de um . Ver mais
{plog:ftitle_list}
Buy and Sell. Discussion. Media. Events. More. About. Buy and Sell. Discussion. Media. Events. Rolinhos de desapegos canguçu. Join group
To carry out the reaction of Zinc with dilute sulphuric acid and classify it as physical or chemical changes. See moreZinc, being more reactive or being higher up in the reactivity series than hydrogen, displaces hydrogen from dilute acids. See more1. Take 5mL of dilute sulphuric acid solution in a test tube. 2. Add zinc granules to it in such a way that zinc pieces are . See more
drop tester dt 202
Zinc reacts with dilute sulphuric acid to produce hydrogen gas (H 2) and zinc sulphate. Zn(s) + H 2 SO 4 (g) → ZnSO 4 (s) + H 2 (Zinc) (dil. sulphuric acid) (Zinc Sulphate) (Hydrogen gas)∙ 16y ago. Best Answer. Zn + H2SO4--> ZnSO4 + H2. The pattern is: acid + metal ---> salt + hydrogen gas. In the video below, pieces of Zn are dropped into a test tube containing dilute.
Activity 1.3 Take a few zinc granules in a conical flask or a test tube. Add dilute hydrochloric acid or sulphuric acid to this (Fig. 1.2).Add 5 cm 3 of dilute sulfuric acid to test tube 3. Then add about 1 cm 3 of the copper sulfate solution using a dropping pipette. Note the rate of production of gas bubbles. Use a lighted splint to test for any gases given off. To the second test tube add a few pieces of iron filings and to the third some zinc turnings. Observe what happens, test for any gases and note down your observations. .
Use the dropping pipette to add no more than 2 cm3 of dilute hydrochloric acid to each test tube. Add the piece of zinc to one of the test tubes. Observe and record any signs of a reaction. If .Add 10 mL of dilute sulphuric acid to a test tube containing a few granules of zinc metal. Fit a rubber cork with a glass tube into the mouth of the test tube. Observe the colour and the odour .
Put a spatula measure of white, anhydrous copper(II) sulfate powder into a test tube. Use a dropping pipette to add a few drops of water to the powder. Watch what happens and feel the .Lesson 1 is a series of test tube experiments in which each working group establishes as a common feature that hydrogen is given off as metals react with an acid – if the metal reacts at .The correct option is D. It is a chemical change, zinc dissolves and hydrogen gas is liberated. Explanation for the correct option: (D) It is a chemical change, zinc dissolves and hydrogen gas is liberated: When 5 mL of dilute Hydrochloric acid (HCl) is taken in a test tube and a few pieces of granulated Zinc (Zn) are added to it, the following chemical reaction takes place. Click here 👆 to get an answer to your question ️ neetu has two test tubes containing dilute hydrochloric acid and dilute sodium hydroxide solution, . Used to pour liquids into containers with small openings or to hold filter paper (unfenl)_____7. Mixing a small amount of chemicals together (lewl letpa)_____8. Heating contents in a test .
Final answer: The element b) Zinc is most likely the metal referred to in the question. It reacts with dilute sulfuric acid to produce hydrogen gas which is colorless and ignites with a match and forms a salt.. Explanation: The metal referred to in the question which, upon adding dilute sulphuric acid, produces a colourless gas when a burning match stick is brought .A zinc + hydrochloric acid -sce results Drop a small piece of zinc into a test tube containing 1-2 ml of 1.0 M hydrochloric acid solution. (products) 1. Complete and balance the equation. 2. Classify the reaction 3. What gas was formed?
A dilute solution of sodium carbonate was added to two test tubes one containing dilute HCl A and the other containing dilute NaOH B. The correct observation was. . A litmus paper was dropped into 2ml dilute HCl. Then 2ml concentrated NaOH was added to it and stirred. . What did the student observe when he added dilute H C l to a test tube .
Give a chemical test to distinguish between : a dilute sulphuric acid and dilute hydrochloric acid, b dilute sulphuric acid and conc. sulphuric acid. . Bring a glass rod dipped in Ammonia solution near the mouth of each test tubes containing dil. HCl and dil. H 2 S O 4 each. . Concentrated H 2 S O 4 gives S O 2 gas with zinc and the gas . Neetu has two test tubes containing dilute hydrochloric acid and dilute sodium hydroxide solution, but they are not labeled. Adding which of the following solutions to the test tubes will help her visually identify the acidic and basic solution? (a) only vinegar (b) only baking soda (c) only sodium chloride (d) either vinegar or sodium chloride A pellet of Zn of mass 10.0g is dropped into a flaskcontaining dilute H2SO4 at a pressure of P=1.00 bar and temperature of 298K. . Given mass of zinc = 10.0 g. Molar mass of zinc = 65.38 g/mol . Mixing a small amount of chemicals together (lewl letpa)_____8. Heating contents in a test tube (estt ubet smalcp)_____9. Holding many test tubes .
Put a few pieces of granulated zinc into each of the three test tubes. Try to have approximately the same amount in each test tube. Add 5 cm 3 of dilute sulfuric acid to test tube 1. Note the rate of production of gas bubbles. Add a few copper turnings to test tube 2. Make sure they are in contact with the zinc. Facebook: https://www.facebook.com/profile.php?id=100016505163491Activity 1.3 Take a few zinc granules in a conical flask or a test tube. Add dilute hydrochloric acid or sulphuric acid to this(Fig. 1.2).CAUTION: Handle t.
Boiling tube, dilute sulphuric acid, NaOH, zinc dust, spatula, dropper, Procedure: Take a boiling tube and add a few drops of dilute sulphuric acid using a dropper and place it in a test tube rack. Then add some zinc dust into the boiling tube using a spatula. In this reaction, zinc reacts with dilute sulphuric acid to form an aqueousClick here👆to get an answer to your question ️ A metal piece is dropped in a test tube containing dil. H2SO4 .When a burning matchstick is brought near the mouth of the test tube, a pop sound produced due to. Solve Study Textbooks Guides. Join / Login. Question .The zinc is oxidised and passes into solution, whilst hydrogen ions are reduced. End products are zinc chloride and hydrogen gas (the latter can be ignited with a lighted taper or match at the mouth of the test tube) . When zinc granules are added to a test tube containing dilute hydrochloric acid then: Q. When dilute hydrochloric acid is .
1. Place a small quantity (about the size of a small pea) of the sodium or potassium salt of each anion into separate small test tubes. Add ten drops of dilute H2SO4 to each solid. CAUTION: Add H2SO: one drop at a time while .
A student was given two test-tubes A and B each containing a colourless solution. When a few drops of universal indicator were added to the two test-tubes, one by one, then the colour of solution in test-tube A changed to blue .Step 2: To test the ___ of the reaction, add ___ drops of solution (from ___ process) into a test tube containing about ___ drops 6.00 M ___. If a ___ solution is obtained, gradually add more ___ dust into the beaker with ___ ___. Repeat this process until the test with ___ gives a ___ solution. Record your observations.
A student added dilute HCl to a test tube containing zinc granules. Which of the following observations are correct? I. Zinc surface became dull and black. II. A gas was evolved which burnt with a pop sound. III. The solution remained colourless.On adding dilute sulphuric acid to a test tube containing a metal ‘X’, a colourless gas is produced when a burning match stick is brought near it. Which of the following correctly represents the metal ‘X’? Place a few pieces of granulated zinc metal in a test tube. Add 2 ml of sodium hydroxide solution and warm the contents of the test tube. This activity needs the teacher’s assistance. Set the apparatus as shown in Figure. Take about 5 ml of dilute sulphuric acid in a test tube and add a few pieces of zinc granules to it.Around 10 drops of 0.1 mol dm-3 barium chloride solution should be added to the first test tube; Around 10 drops of dilute sodium hydroxide solution (NaOH) should be added to the same test tube; Swirl the test tube carefully to mix well; Continue to add sodium hydroxide dropwise to the test tube, until it is in excess
Case: Observe the image given below. There are two test tubes (i) and (ii). Test tube (i) contains a starch solution, while test tube (ii) contains starch solution and saliva. Both the test tubes are kept undisturbed for about 20 min. Later, dilute iodine solution (brown in colour) was added to both the test tubes. A pellet of Zn of mass 10.0 g is dropped into a flask containing dilute H2SO4 at a pressure of P = 1.00 bar and temperature T = 298 K. What is the reaction that occurs? Calculate w for the process. A pellet of Zn of mass 10.0 g is dropped into a flask containing dilute H2SO4 at a pressure of P = 1.00 bar and temperature T = 298 K.Add 10 mL of dilute sulphuric acid to a test tube containing a few granules of zinc metal. Fit a rubber cork with a glass tube into the mouth of the test tube. Observe the colour and the odour of the gas evolved in the reaction mixture. Bring a lit candle near the opening of the glass tube in the mouth of the test tube. Observe the colour of .
Quiz yourself with questions and answers for cpac 11: test tube reactions to identify transition metal ions in aqueous solution, so you can be ready for test day. Explore quizzes and practice tests created by teachers and students or create one from your course material. Zinc pieces are added into dilute H2SO4 taking in a test tube and burning splinter is brought over the mouth o. Get the answers you need, now! vmonika921 vmonika921 04.06.2024 . When zinc granules are added to dilute hydrochloric acid, zinc chloride and hydrogen gas is formed. The reaction involved is:State whether the acid used in each case is dilute or concentrated. Answer (i) Zinc, Iron or any active metal reacts with dilute Sulphuric Acid (H 2 SO 4) to form Hydrogen. The reactions are shown below: . Dense white fumes are produced when a rod dipped in NH 3 soln. is brought near the test tube containing HCl gas. Question 15. Give two .
znso diluted sulfuric acid
web3 de ago. de 2023 · EncourageTV. God. Family. Football. features the rich, diverse personal stories of Evangel’s players, coaching staff, and the broader Shreveport .
dropping zinc pallets into a test tube containing dilute h2so4|zinc and sulfuric acid experiment